June 2023
Campylobacter spp. occur widely as part of the intestinal flora of many warm-blooded animals and birds, particularly cattle and poultry and can be carried in animals that are used for food production and in domestic pets, including cats and dogs. The bacteria have also been found in shellfish. In addition, the bacteria can also occur in untreated water and raw milk.
In UK and Europe, Campylobacter is the most commonly reported bacterial cause of intestinal infection and is considered to be one of the leading global causes of human gastroenteritis. (1) Evidence indicates that the most important risk factors for foodborne infection are consumption of undercooked poultry (particularly chicken) and other meat, unpasteurised milk and food that has been cross-contaminated by raw poultry. Contaminated water or ice has also been shown to be a source of infection.
In 1886, Theodor Escherich observed non-culturable spiral-shaped bacteria in stool samples from children with diarrhoea and subsequently in kittens. These Vibrio spp. (ultimately Campylobacter), were identified for the first time in 1906, when two British veterinarians reported the presence of ‘large numbers of a peculiar organism’ in the uterine mucus of a pregnant sheep. Following this in 1913, McFaydean and Stockman identified Vibrios (Campylobacter) in foetal tissue of aborted sheep. In 1919, Smith studied infectious abortions of cattle and isolated a spiral-shaped bacterium which he rightly associated with the Vibrio spp. reported by McFaydean. He gave the name Vibrio fetus to the organisms and, up until 1962, spiral bacteria were being isolated from farm animals suffering a variety of ailments and called Vibrio.
Meanwhile, in humans, these Vibrios began to be noted as causes of illness. In 1938, over 300 inmates of two prisons in the USA became ill with what is believed to be a milk borne outbreak. Organisms resembling Vibrio jejuni were grown in broth cultures from blood samples of some of those affected.
Sebald and Véron first proposed the genus Campylobacter in 1963, distinguishing them from Vibrio spp. (2), hence Vibrio fetus transferred into a new genus … Campylobacter. Following this, many of the species previously classified as Vibrio were moved into this new genus, including C. jejuni and C. coli.
In 1957, prior to the assignment of the new genus, King (1) and co-workers successfully isolated Vibrios from the blood of humans with diarrhoea - the first ever isolation of them from any source. However, it was only in 1968 that clinical microbiologists in Belgium isolated Campylobacter from stool samples of patients with diarrhoea (3).
The development of selective growth media in the 1970s permitted more laboratories to test stool specimens for Campylobacter, and the species emerged as an important human pathogen.
Campylobacters are the most frequently reported bacterial cause of human diarrhoeal disease in countries where surveillance of infection has been implemented. Campylobacter species are a leading cause of zoonotic enteric infections in many developed countries, commonly affecting infants and children, making Campylobacter a medically and socio-economically important human pathogen. C. jejuni is responsible in developed countries for approximately 90 - 95% of diagnosed Campylobacter infections, C. jejuni causes more than 90%, and C. coli less than 10% of infections in the UK. Other species including C. coli, C. lari and C. upsaliensis may also cause infection. In developing countries C. hyointestinalis may be responsible for a larger percentage of infections.
Within the last 20 years, the reported incidence rate of Campylobacter infections has increased in many countries. The increase in reports may be due in part to increased awareness, improved laboratory diagnosis and surveillance. Under-reporting of Campylobacter infections is believed to be an issue in many countries with a weak reporting structure, with incidence rates only reflecting the number of laboratory-confirmed cases.
Documented outbreaks of Campylobacter infections are rare, with most reported cases being sporadic or as part of small, family-related outbreaks (4,5,6).
Campylobacters, especially C. jejuni, frequently colonise the mucosal layer of the intestinal tract of poultry, other birds and animals. Levels of Campylobacters in the intestines of infected poultry can be particularly high (up to 1010 cfu/g of faeces). During times of stress on the farm the bacteria can be excreted in large numbers, and during slaughter and processing, the poultry carcasses can become contaminated with low numbers of campylobacters released from the intestinal tract during evisceration. A survey of chicken on retail sale in UK showed that 63.1% of chicken skin and 5.5% of outer packaging was contaminated with Campylobacter. An FSA survey (in 2018) found 5.8% of samples from major retailers had Campylobacter present at over 1000cfu/g. (8)
Two genera, Campylobacter and Arcobacter form the family Campylobacteraceae, occur primarily as commensals in humans and domestic animals. The genus Campylobacter generally contains small (0.2 - 0.8 μm by 0.5 - 5 μm) gram-negative, slender spirally curved rods, which form an ‘S’ or a ‘V’ shape when two or more bacterial cells are grouped together. They often join to form zig-zag shapes. The majority of the species have a corkscrew-like motion by means of a single polar unsheathed flagellum at one or both ends of the cell.
C. jejuni has been isolated from cattle, sheep, goats, dogs and cats. In contrast, C. coli is predominantly found in pigs although has also been isolated from poultry, cattle and sheep.
C. jejuni hydrolyses hippurate and indoxyl acetate and reduces nitrate. Most strains are resistant to cephalothin, with resistance to fluoroquinolones becoming an increasing concern, as this is the category of antibiotics normally used to treat animal and human illness. In many European countries, a steady increase in resistance has been seen among Campylobacter isolates with 17 - 99% of Campylobacter strains isolated from humans and animals being resistant to fluoroquinolones (9).

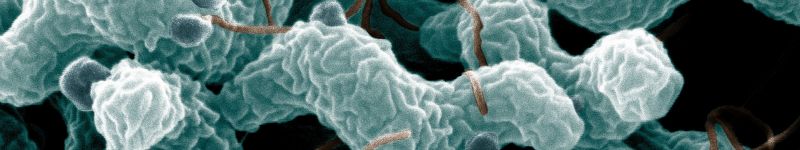
Characteristic spiral or corkscrew shape of C. jejuni cells and related structures (United States Department of Agriculture, Agricultural Research Service).

Image of C. jejuni including flagella (Getty Images)
Campylobacter spp. typically grows at 37°C, but not below 32°C. The high optimum growth temperature of 42°C of C. jejuni and C. coli distinguishes them from most other Campylobacter spp. While it is therefore not likely that Campylobacter will multiply during processing, refrigerated transport and storage, the organism can survive these steps and therefore survive in the refrigerated supply chain.
Campylobacter spp. may persist for prolonged periods in chilled and frozen products. At 4°C a decrease in the concentration and viability of cells has been recorded after several weeks, but the cells can respire, generate adenosine triphosphate (ATP) and move towards more favourable environments. Persistence in frozen poultry may be several months, with the largest log reduction during initial freezing. Oxidative stress has been shown to contribute to the freeze-thaw induced killing of Campylobacter. Research has shown that on chilled raw chicken and pork skin, C. jejuni and C. coli can survive for several weeks.
While most Campylobacter species are microaerophilic, optimally growing in low oxygen or microaerophilic environment (such as an atmosphere of 5% oxygen, 10% carbon dioxide and 85% nitrogen), a few species can grow under strictly aerobic or anaerobic conditions. The microaerophilic species appear to have an inherent sensitivity towards oxygen and its reduction products, with a requirement for cellular defences to survive during exposure to air. Because of this the survival of Campylobacter outside the host intestine is poor and replication does not readily occur outside of the organism. C. jejuni may be able to adapt to differing hosts and niches and survive environmental atmospheric oxygen conditions (13). The organism is sensitive to heat, drying and acidic conditions (pH of less than 4.9 with optimal growth at pH 6.5 to 7.5) and salinity. Campylobacter is sensitive to salt concentrations above 1.5%; both C. jejuni and C. coli are sensitive to heat and do not survive cooking or pasteurisation temperatures with D-values of 0.21 - 2.25 minutes at 55 -60°C. Research has shown that C. jejuni can mount adaptive responses to both acidic and aerobic conditions, and there is increasing recognition that Campylobacter is more resistant to stress than had initially been thought. The organism is relatively sensitive to osmotic stress, however evidence shows that the organism can be recovered from dry surfaces 24 hours after contamination, albeit in low numbers.
An important factor in the persistence of the organisms is their tendency to become attached to or entrapped in the skin surface and this may also limit the removal of Campylobacter during carcass washing. This tendency seems to offer a degree of protection to the organism from environmental stresses encountered during heating, chilling and exposure to chlorinated water. Campylobacter is unable to multiply in the processing plant, as the minimum growth temperature is 32 - 35°C and the optimum is 37 - 42°C. Growth outside the intestine requires a reduced concentration of atmospheric oxygen and preferably 10% carbon dioxide. C. jejuni may survive at 4ºC in low oxygen, moist foods for 2 to 4 weeks.
Under unfavourable growth conditions, such as osmotic stress, Campylobacter converts from the typical spiral shape to a coccoid form, possibly via a ‘doughnut’ shape as the cells curl during the transition from rod to coccoid shaped. This form is non-motile, despite maintaining the presence of flagella, and is likely to be a non-viable degenerate cell form which is undergoing an autolytic process of cellular degradation, with a possible role in biofilm formation (10,11). Research has shown that Campylobacter species can survive cleaning and disinfection steps in poultry processing environments and survive for at least 21 days (11).
The gastrointestinal tracts of birds (particularly poultry), cattle, rodents and domestic pets are typical reservoirs of infection for Campylobacter. C. jejuni grows best at the body temperature of a bird and seems to be well adapted to birds, which carry it without becoming ill. Flies are a vehicle for this organism through transmission from faecal sources to broiler chickens (18). Some wild birds including the European Blackbird may be a persistent source of Campylobacter (19). As already discussed, poultry can have a high bacterial load and occupational exposure when processing poultry in abattoirs may be implicated in some cases. While not usually spread from person to person, this can happen if the infected person is producing a large volume of diarrhoea and personal hygiene is poor.
For consumers, the primary reservoir is typically undercooked meat (especially poultry) or cross contamination, such as using the same equipment and utensils for raw poultry and vegetables, or other raw or lightly cooked products. A considerable proportion of raw chicken on sale in the UK is contaminated with a high level of Campylobacter (7). Barbecued meat, bottled mineral water, unpasteurised milk, bird-pecked milk in bottles on doorsteps, and untreated water are all vehicles of Campylobacter. Young children acquire the infection through contact with leakage from poultry packages in shopping trollies, and cases have been reported from contact with stools of an ill dog or cat.
Other risk factors that have been identified are acquiring an infection during travelling, contact with pets and farm animals, recreational activities (e.g. sand from beaches, surface waters, rivers and lakes) and in nature via sewage and from the faeces of wild animals and birds.
Infectivity and symptoms
The main route of infectivity is generally foodborne, with a low infectious dose (500 or less cells) for Campylobacter dependent upon a number of factors which may be strain-specific, including the virulence of the strain, the vehicle with which it is ingested and the susceptibility of the individual, although the organism does not multiply in food. The most common symptoms of campylobacter infection include diarrhoea (from profuse and watery, to bloody and dysenteric), abdominal pain which can mimic acute appendicitis, fever, headache and nausea, with vomiting being uncommon, but not unknown. Symptoms usually start 2 to 5 days after infection, although can be 1 to 11 days after infection, lasting for 3 to 6 days, although mild relapses do occur and the organism may continue for up to 2 to 3 weeks (11).
The elderly, children, and individuals with immuno-compromised illnesses are most at risk. Due to contaminated aerosols, new workers at slaughterhouses also have an increased risk. Young adults (aged 15 to 25 years old) appear to be either more frequently exposed or more susceptible to Campylobacter than other age groups. The reason is unknown but males appear to have a higher incident rate of infections than females.
Specific treatment is not usually necessary, except to replace electrolytes and water lost through diarrhoea. Antimicrobials may be needed to treat invasive cases and the carrier state. Severe complications, such as reactive arthritis, Guillain-Barré syndrome (GBS), a demyelinating disorder of the peripheral nervous system resulting in weakness of the limbs, weakness of the respiratory muscles and loss of reflexes and Miller-Fischer Syndrome (thought to be a variant of GBS) may follow Campylobacter infection. Few deaths are related to Campylobacter infections but typically occur among the at-risk populations of infants, elderly and immuno-suppressed individuals.
A vital part of the pathogenesis of Campylobacter-mediated gastroenteritis is the penetration of the intestinal mucosa. After adhering to the intestinal cell lining, the bacterium becomes internalised within the cells, reflecting the virulent mechanism by which the organism gains access to sub-mucosal tissue causing damage and inflammation and leading to gastroenteritis (13). Campylobacter adheres to cell membranes and is internalised into cytoplasmic vacuoles, providing the saccharolytic, slow-growing organism a suitable intracellular niche, where microbial competition is either less or absent, and Campylobacter can process metabolites from other microbes.
Each year the reported number of cases of Campylobacter regularly changes, with a consistent increase in the late spring and early summer (particularly in children under 5), which correlates with an increase in Campylobacter bacterial load in broiler chicken flocks and raw poultry in retail, then studies also show a gradual reduction during the summer and autumn months. The changes in cases since 1989 have been broadly similar for most regions in the UK with an increase between 1987 and 2001. Some of the changes up to 2008 may reflect improvements in the diagnosis and reporting over that period. 2019 data shows 56,439 reported cases of Campylobacter in England, an increase of 161 cases. The region that reported the highest number was the South East with 11,135 reports. Overall, 56% of Campylobacter laboratory-confirmed cases in England were male. Those in the age range 50 to 59 years had the highest number of cases, and July was the peak month.
Seasonality of laboratory reports of all Campylobacter species in England

Outbreaks are infrequent but large outbreaks from raw and inadequately pasteurised milk and contaminated water supplies have been reported, with numbers increasing since 2009. Campylobacter is now the most frequently implicated causative agent causing 24% of all reported outbreaks (11). Cases in England and Wales increased from 1989 to 2000, declined between 2000 and 2004 and rose again since 2004 (9). In 2016 there was a 17% decrease in the number of laboratory reports reported by UK surveillance bodies, however since then the number of reported cases has remained around 55,000 per annum.
Annual laboratory reports of Campylobacter (in England, 2010 to 2019)

General outbreaks of Campylobacter are rare, with 0.4% of outbreaks from 1995 to 1995 (7). Most outbreaks were associated with consumption of undercooked poultry liver pâté, or parfait, from food service establishments, with the remainder from residential or institutional settings (11). The 2019 data shows two outbreaks with 37 people affected, both being linked to food service environments and one with chicken liver pâté.
Raw poultry handling and the consumption of poultry products have typically been identified as important risk factors, accounting for a variable percentage of cases, along with cross contamination of Campylobacter from raw chicken to prepared food. Other food-related risk factors that have repeatedly been identified include consumption of other meat types, undercooked or barbecued meat, raw seafood, drinking untreated surface water, contaminated cucumbers or unpasteurised milk or dairy products. Eating meat cooked outside the home (at restaurants), has also been identified as a risk factor in USA and New Zealand.
While Campylobacter does not multiply within food, the bacteria rapidly attach to a surface when exposed to air and encase themselves in a sticky biofilm. Cells can be shed from the biofilm and potentially enter the food chain. This provides an example of its ability to sense and respond quickly to stresses in its environment and may have an impact on how infections are spread (15).
Due to the varying growth requirements of different species, detection and isolation of Campylobacter from foods and food-animal matrices is dependent on the types of media, isolation methods and laboratory method used. Primary isolation of this organism usually requires the use of selective filtration, non-selective media and incubation at 37°C. These methods typically require an enrichment step and isolation on one or more selective agar media, resulting in either a negative result, or a presumptive positive, which requires confirmation.
The various species do not ferment or oxidise sugars and are sensitive to hydrogen peroxide and superoxide anions produced in media. To increase the aerotolerance of the organisms, and neutralise the toxic products of oxygen, various oxygen scavengers can be added to enrichment broths and selective agars, including superoxide dismutase, catalase, lysed blood, FBP (0.025% each of ferrous sulphate, sodium metabisulphite and sodium pyruvate) or CFP (0.4% charcoal, 0.025% ferrous sulphate and 0.025% sodium pyruvate). Oxygen sensitivity is dependent on the growth media so results obtained in one substrate do not necessarily translate to others.
Alternative and rapid methods have been developed for the detection and confirmation of the presence of Campylobacter spp. with examples including fluorescence in situ hybridisation, latex agglutination and a physical enrichment method (filtration) that permits the separation of Campylobacter from other organisms present in the food matrix. Polymerase chain reaction (PCR) reaction tests can be very effective and this method has been combined with some success with immuno-separation giving the ability to detect low numbers in around 6 to 8 hours.
It is recommended that any company wishing to use a rapid detection method should only consider those that are well validated (for example by MicroVal, AFNOR, NordVal or AOAC) and they should also check that these methods work with their own ingredient and product types.
Taking this approach should achieve a microbiological load generally viewed as safe. For more details refer to the IFST Handbook of Microbiological Criteria for Foods (Purchase here: Handbook of Microbiological Criteria for Foods | IFST)
Farms
- High levels of on-farm biosecurity are paramount and research on model farms suggests that near-perfect conditions, and consistent application of working practices, may give a 10% reduction in the number of flocks infected by Campylobacter
- Effective biosecurity can be achieved by the application of disinfectant foot dips, changing boots and clothing and physical barriers to stop dirt from entering from outside are important elements. Good standards of maintenance and pest proofing, including fly screens, are also key. It is important that buildings are of sound construction and well maintained to prevent access by wild birds, insects and to deter rodents
- Chlorination of the water supply (2ppm) or in-line UV treatment
- Biosecurity and intervention measures including hygiene practices to prevent introduction from external sources, by farm staff, such as dedicated clothing and footwear
- Cleaning and disinfection of barns and houses between flocks
- Preventing access of cats and dogs to poultry flocks
- Limit ‘thinning’ of flocks as it may introduce Campylobacter through staff, crates and modules and also cause stress, resulting in increased susceptibility to colonisation, or carry out thinning in association with crate washing to reduce microbial load and biosecurity measures
- Wash crate surfaces and lorry decks in a manner that will reduce Campylobacter load e.g. warm/hot water, suitable detergents and or/disinfectants at correct usage levels between journeys, including lorry wheels
- High levels of stockmanship and staff training on flock infection
- Simple modifications of the diet, e.g. addition of organic acids or probiotics, has shown a potential influence upon Campylobacter levels with no net adverse effects on the health, welfare or productivity of the animal.
Animal transport
- Animal welfare handling practices to reduce stress.
Slaughter and processing
- Hazard Analysis and Critical Control Point (HACCP) and up-to-date Good Manufacturing Practice (GMP) practices
- Segregation of Campylobacter-positive flocks from negative flocks for poultry (using rapid-testing protocols) at slaughterhouses, and slaughtering positive flocks
- Withdrawal of feed for at least 12 hours before slaughter.
Scalding
- Moving carcasses against the flow of incoming water (counter-flow) so that they meet the cleanest water at the carcass-exit end
- High water flow rates of water with adequate agitation
- Optimum scalding temperature to minimise levels of Campylobacter
- Use of approved chemicals e.g. pH regulators
- Using several successive tanks operated in the same way to provide a dilution effect
- Empty and clean tanks at the end of a processing period (at least twice daily)
- Hygiene measures applied to reused/recycled water.
Evisceration
- Minimise rupture of exposed intestines and prevent the spread of faecal bacteria, such as Campylobacter, which occur in relatively high numbers, particularly in the intestines of positive birds
- Careful siting of evisceration machinery to prevent microbial attachment and cross contamination
- Strategic washing of carcasses at points close to sites of contamination, in order to avoid microbial attachment
- Spraying contact surfaces with chlorinated water where legislation allows
- Use of automatic transfer of carcasses from the slaughter line to the evisceration line and, where possible, on to the chilling line.
Washing
- High-pressure washers may remove significant numbers of microbial contaminants from both the inner and outer surfaces of the carcass. The extent to which contamination is reduced at this stage depends upon the frequency and degree of washing at earlier stages, but may have little effect on cells attached to carcass surfaces. Research has shown that carcass washing equipment, operating at a consistently high efficiency, can reduce the amount of Campylobacter by up to 10%.
Chilling/freezing
- Super-chlorination of chilling water is of value in controlling cross-contamination via the water itself, although is thought to have little further effect on microbial contamination of carcasses
- Processing aids including free chlorine, organic acids and other antioxidants added to potable, chilled water or recirculated water
- Storage at -20°C for at least 31 days
- Crust freezing for skinless, boneless cuts through continuous carbon dioxide belt freezing.
Processing
- Heat treatments: Campylobacter is sensitive to heat and cold, hence such interventions are being used by some in the industry. Both must be balanced with the need to maintain the appearance of the meat that is acceptable to the consumer. One example is SonoSteamTM , an intervention that uses a combination of ultrasound and steam to reduce the number of microorganisms on the surface of the bird
- Chill treatments: rapid surface chilling is where nitrogen, or air, at a very low temperature, is sprayed onto the bird to lower the surface temperature and reduce Campylobacter, such as the one developed by BOC Ltd. Trials demonstrated a 1 to 1.5 log reduction
- Novel packaging: there has been significant development of roast-in-the-bag whole chickens which removes the need to handle the bird and aims to reduce incidences of cross-contamination, and helps consumers have good kitchen hygiene practices. The bag containing the chicken is placed in the oven, then cut open a short time before the end of the cooking time to allow the meat to brown
- Use of GMP to prevent cross-contamination from workers, tools and equipment
- Risk management to differentiate between Campylobacter from the carcass exterior and those in the faeces that leak during evisceration
- Treatment of municipal water supplies
- Consumer education and cooking instructions
- Pasteurisation of milk.
[1] International Food Hygiene Microbial Update: Campylobacter (thermoscientific.com)
[2] International Journal of Systematic and Evolutionary Microbiology doi: 10.1099/00207713-23-2-122 May 2006 vol. 56 no. 5 937-945
[3] Altedruse SF, Stern NJ, Fields PI, Swerdlow DL. Campylobacter jejuni—An Emerging Foodborne Pathogen.Emerg Infect Dis [serial on the Internet]. 1999, Feb
[4] Available from http://wwwnc.cdc.gov/eid/article/5/1/99-0104.htm
[5] Hilpi Rautelin. Campylobacter, The Lancet Infectious Diseases, Volume 8, Issue 11, Page 673
[6 & 7] Campylobacter (who.int)
[9] Blaser MJ, Engberg J. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3. ASM Press; Washington DC, USA: 2008. pp. 99–121.
[10] Silva J, Leite D, Fernandes M, Mena C, Gibbs PA and Teixeira P (2011) Campylobacter spp. as a foodborne pathogen: a review. Front. Microbio. 2:200. doi: 10.3389/fmicb.2011.00200
[11] Eur J Microbiol Immunol (Bp). 2012 Mar; 2(1): 41–49. Published online 2012 Accessed: Mar 2017. DOI: 10.1556/EuJMI.2.2012.1.7 PMCID: PMC3933989. Putative mechanisms and biological role of coccoid form formation in Campylobacter jejuni. N. Ikeda and A. V. Karlyshev
[12] Autolytic process (J Clin Microbiol. 1983 August; 18(2): 420–421. PMCID: PMC270816 Electron microscopy of the coccoid form of Campylobacter
jejuni. G E Buck, K A Parshall, and C P Davis )
[13] Food Microbiol. 2017 Aug;65:185-192. doi: 10.1016/j.fm.2017.02.009. Epub 2017 Feb 24. Campylobacter jejuni survival in a poultry processing plant environment. García-Sánchez L, Melero B, Jaime I, Hänninen ML, Rossi M, Rovira J.
[14] Front Microbiol. 2016 Dec 26;7:2117. doi: .3389/fmicb.2016.02117. eCollection 2016. The Campylobacter jejuni Oxidative Stress Regulator RrpB Is Associated with a Genomic Hypervariable Region and Altered Oxidative Stress Resistance. Gundogdu O, da Silva DT, Mohammad B, Elmi A, Wren BW, van Vliet AH, Dorrell N.
[17] Campylobacter Virulence Factors and Molecular Host-Pathogen Interactions - PubMed (nih.gov)
[18] J Food Prot. 2003 Sep;66(9):1587-94. Survival and persistence of Campylobacter and Salmonella species under various organic loads on food contact surfaces. De Cesare A, Sheldon BW, Smith KS , Jaykus LA
[20] Epidemiol Infect. 2016 Nov;144(15):3326-3334. Epub 2016 Aug 15. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Royden A, Wedley A, Merga JY, Rushton S, Hald B, Humphrey T, Williams NJ.
[21] Environ Microbiol Rep. 2015 Oct;7(5):782-8. doi: 10.1111/1758-2229.12314. Epub 2015 Sep 8.Wild bird-associated Campylobacter jejuni isolates are a consistent source of human disease, in Oxfordshire, United Kingdom. Cody AJ, McCarthy ND, Bray JE, Wimalarathna HM, Colles FM, Jansen van Rensburg MJ, Dingle KE, Waldenström J, Maiden MC.
Links
[1] http://wwwnc.cdc.gov/eid/article/5/1/99-0104.htm
[2] http://www.who.int/topics/campylobacter/en/
[3] Detection and enumeration of Campylobacter species (publishing.service.gov.uk)
[4] BAM Chapter 7: Campylobacter | FDA
[5] International Food Hygiene Microbial Update: Campylobacter (thermoscientific.com)
Further reading
[1] Campylobacter - Features, Detection, and Prevention of Foodborne Disease
1st Edition - September 13, 2016 Editor: Günter Klein eBook ISBN: 9780128036495 Paperback ISBN: 978012803623
Institute of Food Science & Technology has authorised the publication of this Information Statement on Foodborne campylobacteriosis.
This is an update to the Information Statement that was originally prepared by Julie Ashmore CSci FIFST in 2017. It has been peer-reviewed and approved by the IFST Scientific Committee and is dated June 2023.
The Institute takes every possible care in compiling, preparing and issuing the information contained in IFST Information Statements, but can accept no liability whatsoever in connection with them. Nothing in them should be construed as absolving anyone from complying with legal requirements. They are provided for general information and guidance and to express expert professional interpretation and opinion, on important food-related issues.
